Elements, Compounds, and Mixtures
- Grade Levels
- Related Academic Standards
- Cess Anchors
- Eligible Content
- Big Ideas
- A technological world requires that humans develop capabilities to solve technological challenges and improve products for the manner we live.
- Each expanse of technology has a ready of characteristics that separates information technology from others; still, many areas overlap in gild to meet human needs and wants.
- Technological design is a creative process that anyone tin do which may result in new inventions and innovations.
- Technological literacy is the power to apply, appraise and manage technology effectually us.
- Technology is created, used and modified past humans.
- Concepts
- A technological blueprint & trouble solving process changes ideas into a terminal product or arrangement.
- Bio-related technologies are the processes of using biological mater to brand or modify products.
- Bio-related technologies are the processes of using biological organisms to make or change products.
- Communication is the procedure of composing, sending, and receiving letters through technology.
- Communication is the process of composing, sending, and receiving letters using technological devices.
- Structure is the process of turning materials into useful structures.
- Construction is the process of turning raw materials into useful structures.
- Decisions about the use of products and systems tin result in expected and unexpected consequences.
- Energy and power technologies are the processes of converting energy sources into useful power.
- Energy and power technologies use processes to convert free energy into power.
- In a technological globe, inventions and innovations must exist carefully assessed past individuals and society equally a whole.
- Innovation is the process of improving an existing product, process, or organisation.
- Innovation is the process of modifying an existing production, process, or system to improve it.
- Invention is a process of creating new products, processes, or systems.
- Invention is a process of turning ideas and imagination into new products, processes, or systems.
- Inventions and innovations must be carefully assessed past individuals and gild.
- Manufacturing is the procedure of turning materials into useful products.
- Manufacturing is the process of turning raw materials into useful products.
- People select, create, and utilize scientific discipline and technology and are limited by constraints (e.g. social and physical).
- People select, create, and use technology.
- Safe is a preeminent business concern for all technological development and utilise.
- Safety is one of the most of import concerns for all technological evolution and use.
- Scientific discipline and technology are interconnected.
- Technological blueprint & problem solving follows many steps.
- Technological blueprint & problem solving includes clearly communicated solutions.
- Technological pattern & trouble solving includes frequent checking.
- Technological design & problem solving requires easily-on applications.
- Technological literacy is a lifetime endeavor.
- Technological literacy is necessary for a productive 21st century skilled workforce.
- Technological literacy is necessary for a productive workforce.
- Technological literacy is necessary for all citizens.
- Technological literacy is required for all citizens in a democratic order for shared decision-making.
- Technological literacy is the ability to understand, use, assess, design, and create technology.
- Technological literacy is the ability to sympathize, apply, assess, design, and produce technology (i.due east. Invention & Innovation).
- Technological literacy requires lifelong learning.
- Technology and society impact each other.
- Technology and society mutually touch on each other.
- The abilities required in a technological globe include diagnosing, troubleshooting, analyzing and maintaining systems.
- The abilities required in a technological world include understanding, fixing, and maintaining systems.
- The goal of technology is to run across human needs and wants.
- Transportation is the process of safely and efficiently moving people and products.
- Understanding technological systems aid usa plan and control technological developments.
- While scientific discipline is the study of the natural globe, technology is the study of the human designed world.
- Competencies
- Create a new product, process, or system.
- Describe and demonstrate how to use technological design & problem solving.
- Describe how scientific discipline and applied science piece of work together.
- Pattern and develop the ability to create and send messages using technological devices.
- Design and develop the ability to safely and effectively utilise tools and materials to build structures.
- Design and develop the ability to safely and finer use tools and materials to catechumen energy into power.
- Blueprint and develop the power to safely and effectively use tools and materials to create bio-related products and systems using technology.
- Design and develop the power to safely and effectively utilise tools and materials to create vehicles that ship people and products.
- Design and develop the ability to safely and effectively employ tools and materials to industry products.
- Design and produce solutions to technological problems.
In this lesson, students will learn that all thing can be classified as either a pure substance or a mixture. They volition besides learn that both mixtures and pure substances can be broken down into subcategories and that there are techniques chemists utilize to make up one's mind in which category a sample of matter belongs. Students will:
-
classify a sample of matter in terms of pure substances and mixtures.
-
distinguish betwixt homogenous and heterogeneous mixtures.
-
distinguish between solutions, colloids, and suspensions.
-
recognize the difference between an element and a chemical compound.
-
Element: Pure substance consisting of i type of atom.
-
Compound: Pure substance consisting of two or more different atoms.
-
Mixture: 2 or more different substances not chemically combined.
-
Colloid: A heterogeneous mixture that exhibits the Tyndall effect.
-
Suspension: A heterogeneous mixture that has particles large enough to settle out.
-
Solution: A homogenous mixture in which the particles are very modest.
-
Tyndall issue: The scattering of light in a colloid.
-
Homogeneous mixture: A mixture with a uniform composition.
-
Heterogeneous mixture: A mixture with a nonuniform composition.
-
Element symbol: An abridgement for an element's proper name constitute on the periodic table.
-
Compound formula: Represents the combination of two or more elements in fixed proportions. Subscripts designate the number of atoms of each element.
90 minutes/ii class periods
Prerequisite Skills haven't been entered into the lesson plan.
-
pictures of pizza, h2o, mercury, blood, and cola
-
board, markers
-
laser arrow
-
yard mL flasks/beakers (2)
-
10 to 20 drops of milk
-
water
-
Mixtures vs. Pure Substances–Teacher (South-eight-5-2_Mixtures vs. Pure Substances Teacher.medico)
-
Mixtures vs. Pure Substances–Student (Southward-8-5-2_Mixtures vs. Pure Substances Student.doc)
-
Tyndall outcome photo: http://www.silvermedicine.org/dark_tyndal_with_h2o2.jpg
Related Unit and Lesson Plans
- Matter Matters
- Physical Properties of Matter
- Separation of a Mixture
Related Materials & Resources
- View
Students can be assessed on this lesson in the following ways:
-
After explaining the difference between elements and compounds, have students classify a list of pure substances. This will provide an opportunity to reteach the differences and similarities between elements and compounds.
-
Both students and instructor tin monitor understanding of the lesson with the Mixtures vs. Pure Substances lab activity. Students will be able to revisit an earlier activity and apply their caused knowledge. This action allows for revision and reflection.
Suggested Instructional Supports
- View
Scaffolding, Explicit Instruction
| W: | -
The chief focus of this lesson is to be able to categorize all matter. Students larn the difference between a pure substance and a mixture. They will then be able to categorize a mixture as either a colloid suspension or solution. They are evaluated formatively based on their responses during guided pedagogy. They are formally evaluated on the worksheet. |
| H: | -
The lesson begins with several pictures of everyday objects that students are asked to categorize. They revisit their answers at the stop of the lesson to see which answers were correct and which need to exist revised. |
| E: | -
The more than you can relate categorizing to real-life, everyday objects, the more students will see the chemistry connectedness and the importance of categorizing matter. |
| R: | -
Responses to pupil questions need to be more than simply yes/no answers. Students will apply lesson cloth upon completion of the worksheet. They also revisit the pictures at the start of the lesson. |
| E: | -
The worksheet at the end of the lesson provides an opportunity for students to show what they accept learned. They are given fourth dimension to reverberate and revise their initial responses to v photos. |
| T: | -
At the beginning of the lesson, when students are asked to categorize everyday objects, they could work in minor teams. Additionally, molecular-level representations are provided equally extensions, which may back up visual learners. |
| O: | -
This lesson is organized so that in the get-go students are asked to perform a chore using their prior cognition. The lesson moves to teacher-guided instruction, with demonstrations included. The lesson then asks students to revisit the determinations they made in the outset, this fourth dimension with more than noesis. Finally, students consummate a summary worksheet. |
- View

Testify the pictures of common mixtures and pure substances such as:
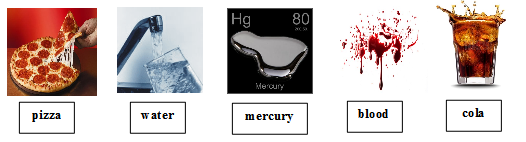
Inquire students to classify each photo equally either a pure substance or a mixture. Write their answers downwardly nether each photo. Tell them that you will revisit these pictures at the end of the lesson and run across if their answers change.
Tell students, "In the last lesson you learned that all matter can be described using physical backdrop. We will at present look at specific categories in which all matter tin exist classified. In that location are two major categories that encompass all matter: pure substances and mixtures." Put the following definitions on the lath:
-
Mixture: 2 or more different substances that are not chemically combined and can be physically separated.
-
Pure substance: A substance that cannot be physically separated.
Say, "Tin can anyone think of an case of a mixture?" If students give a pure substance as their answer, explain that it would not exist a mixture because one cannot carve up it using physical means. Tell them there are two types of mixtures:
-
Homogeneous mixture: A mixture with a uniform composition.
-
Heterogeneous mixture: A mixture with a nonuniform limerick.
Give examples of homogeneous mixtures, including: cola, coffee, and iced tea. Inquire, "What do cola, java, and iced tea have in common? Focus on the mode they wait, rather than their function or ingredients." Guide them to find that all three homogenous mixtures look compatible throughout. They cannot see whatever particles floating or sinking. Say, "Notice that they all await akin. But by looking at them, you may not fifty-fifty know they are made from more than one substance." Follow that with, "If cola, java and iced tea are all examples of homogenous mixtures, what would a heterogeneous mixture look similar?" Students may offering that heterogeneous mixtures will wait similar they are made of different substances. Say, "What about Clot-O? It looks uniform. Would it surprise you to know that Clot-O is not a homogeneous mixture? It is heterogeneous! We demand to dive into more specific descriptions virtually mixtures. Heterogeneous and homogenous mixtures have subgroups. All homogenous mixtures are called solutions. Heterogeneous mixtures can be either colloids or suspensions." The general rules are:
Demonstration
Students may have a difficult time understanding the Tyndall effect. A sit-in works to convalesce this problem. Fill up a flask with 1000 mL of h2o. Add 10 to 20 drops of milk. With the lights off, smooth a laser (generic laser pointers work) through the flask. They will see the laser get through the colloid. This is proof that colloids scatter light. Do the same process with plain water. They will not see the laser lite within the liquid.

http://www.silvermedicine.org/dark_tyndal_with_h2o2.jpg
Say, "Now that we have talked virtually mixtures, what about the other category of matter? Pure substances can also be further described as either compounds or elements." Ask students to give you examples of elements. Refer them to the Periodic tabular array. Define element on the board.
-
Element: Pure substance consisting of one type of atom.
-
Chemical element symbol: An abridgement for an element's name, found on the Periodic table.
Inquire, "What practise you have if you have more than one type of element?" Define compound on the board.
-
Compound: Pure substance consisting of two or more unlike atoms.
-
Chemical compound formula: Represents the combination of two or more elements in stock-still proportions. Subscripts designate the number of atoms of each element.
Put the post-obit listing on the board and ask students to categorize each equally either an chemical element or a compound:
For students who might demand additional practice, reinforce that compounds must have two or more different elements.
Lab Activity
- Use the Mixtures vs. Pure Substances–Teacher sheet (Southward-8-5-2_Mixtures vs. Pure Substances Teacher.physician) to set upwardly the Mixtures vs. Pure Substances lab and to correct and assess students' piece of work when they have finished the lab. Assign students to teams. Hand out Mixtures vs. Pure Substances–Student (South-8-5-2_Mixtures vs. Pure Substances Student.medico) to students. In their groups, they should consummate the information table for the examples, giving a designation and reason for each.
Extension:
-
For students performing above and beyond the standards, have them fill up out a flowchart similar to the one shown at http://www.shschem.info/Classifying%20Matter.htm to assist them organize information throughout the lesson.
-
Students requiring more than practice with the standards may detect it helpful to express the difference between a homogenous and heterogeneous mixture on a molecular level, every bit shown below. Students tin can employ the pictures throughout the lesson every bit a reference or if needed, limited answers and definitions in movie form, equally shown below.
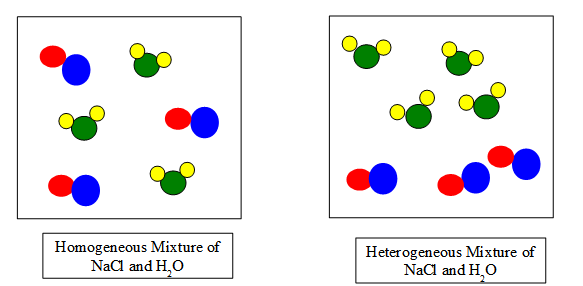
Related Instructional Videos
Note: Video playback may non work on all devices.
Instructional videos haven't been assigned to the lesson plan.
0 Response to "Which Pure Substance Can Be Classified as an Element?"
Post a Comment